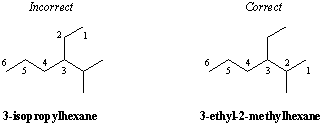
Classification of Carbon Substitution
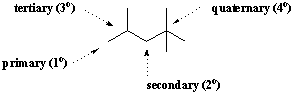
A particular carbon atom is often described in terms of its degree of branching. When a carbon is attached to only one other carbon atom, it is said to be primary(1o). Similarly carbons attached to two, three, and four other carbon atoms are secondary(2o), tertiary(3o), and quaternary(4o), respectively. Methane is not attached to any other carbons, so it forms its own category in this classification system.
Nomenclature of Alkenes and Alkynes
Alkenes and alkynes are named with the same prefixes as their alkane counterparts but their suffixes are changed to -ene and - yne, respectively. The position of the double or triple bond within the carbon chain is denoted by the position of the carbon within the bonded pair that has the lower numbering. The numbering of the parent chain should also be oriented in such a way that the double bond receives the lowest numbering possible: A hexene with its double bond at the end should be 1-hexene, not 2-, 5-, or 6-hexene.
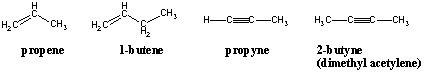
Alkenes have a general molecular formula CnH2n and alkynes have a genera...molecular formula of CnH(2n - 2). This trend makes sense because the presence of each pi (Π) bond removes two σ bonds available for bonding to hydrogens. We will see that there are chemical reactions that add hydrogens to C-C Π bonds and turn alkenes and alkynes into alkanes, and that there are reactions to reverse the transformation and produce alkenes and alkynes from alkanes. An alkane is said to be a saturated hydrocarbon because no more hydrogens can be added to the molecule. Conversely, alkenes and alkynes are unsaturated hydrocarbons. The number of pairs of hydrogens that a hydrocarbon is missing from (2n + 2) is its unsaturation number. A molecule's unsaturation number can be calculated from its molecular formula CnHm:
UnsaturationNumber = 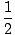 ((2n + 2)–m) ((2n + 2)–m) |
Even though it's impossible to add hydrogens to cyclic alkanes, cyclic alkanes are still considered "unsaturated" in this sense because they have molecular formulas CnH2n. In general, a molecule's unsaturation number is equal to the sum of its number of Π bonds and rings.
Cis-trans Isomerism in Alkenes
Other types of isomerism exist besides constitutional isomerism. Two molecules can have the same atomic connectivities and yet have different spatial arrangements of atoms. Such isomers are stereoisomers. Stereoisomerism takes many forms and will be discussed in great detail in the next chapter.
Alkenes exhibit one form of stereoisomerism. To understand how alkenes can form stereoisomers, recall that the C=C double bond consists of a σ bond between the atoms and a Π bond that lies above and below the plane of the molecule. The strength of the Π bond depends directly on the degree of physical overlap between adjacent p-orbitals. This implies that it is impossible to rotate about the double bond without breaking the Π bond completely. This requires a great deal of energy and does not occur to any appreciable extent at room temperature.
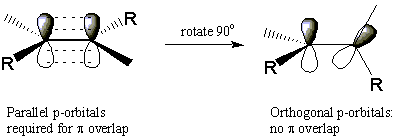
This lack of rotational freedom explains why the following two molecules cannot readily interconvert. These two molecules are stereoisomers because they have the same atomic connectivity and yet are different. The isomer in which both methyl substituents are on the same side of the double bond is called cis, meaning "same". The other isomer with substituents on opposite sides of the double bond is called trans, which means "across". This particular type of stereoisomerism is called cis-trans isomerism, or geometrical isomerism. As we'll see, cyclic alkanes can also exhibit cis-trans isomerism.


 payment page
payment page



