Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews December 29, 2024 December 22, 2024
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Nomenclature and Isomerism
In n-alkanes, no carbon is bonded to more than two other carbons, giving rise to a linear chain. When a carbon is bonded to more than two other carbons, a branch is formed. The smallest branched alkane is isobutane. Notice that isobutane has the same molecular formula, C4H10, as n-butane but has a different structural formula. Two different molecules which have the same molecular formula are isomers. Isomers which differ in the connectivity of bonds are constitutional isomers, or structural isomers. Isobutane is a constitutional isomer of n-butane. The prefix "iso" indicates that branches off of the central carbon are equivalent.

n-butane and isobutane are the only constitutional isomers of C4H10. Pentane, C5H12, has three while hexane, C6H14, has five.
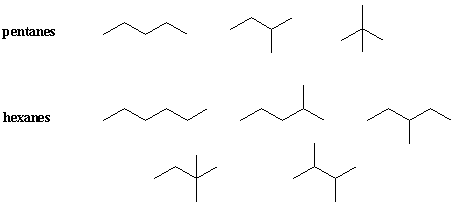
Isobutane, neopentane, etc. are trivial names that arise from common usage. As you can see, the number of isomers increases rapidly for larger alkanes. It would be impractical to give trivial names to every isomer. What is needed is a systematic, easy-to-use method of naming that works for even the most complex of molecules. Such a name should unambiguously identify the structural formula of the named molecule. This system is IUPAC nomenclature, devised by the International Union of Pure and Applied Chemists.
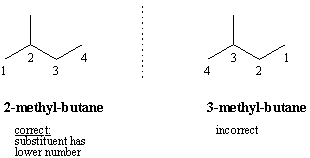
The IUPAC system considers molecules in terms of a parent hydrocarbon chain with substituents attached to it. The parent is the longest continuous carbon chain in the compound, and the base name of the compound is the alkane that corresponds to the parent chain. Then, consecutively number the carbons of the parent chain in such a way that the substituents are attached to carbons with lower numbers. The name of the compound is the parent alkane prefixed by its substituents and their position numberings.
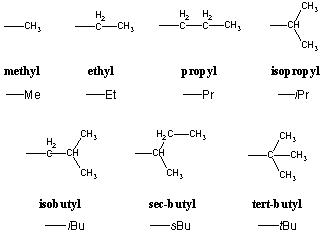
The -CH3 group is called a methyl group. In general, alkyl substituents are derived from the corresponding alkanes by replacing the -ane suffix with -yl. These substituents are used so frequently that they are given abbreviated names. For instance, methyl groups can be abbreviated as -Me. In some instances, the exact nature of the substituent is unimportant. In such cases the notation -R can be used to denote a radical group, a general substituent that can be any organic component.
Sometimes there is more than one possible choice of parent chains. In such cases, choose the parent chain whose substituents are least substituted.
Please wait while we process your payment

