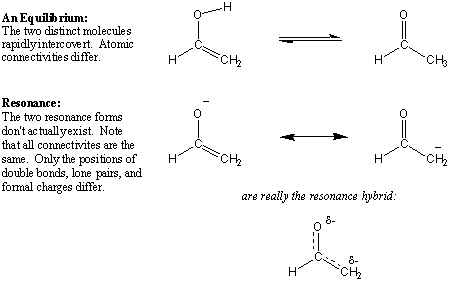
Resonance vs. Equilibrium
Do not confuse the double-headed arrows that denote resonance with the two
single-headed arrows that denote equilibrium. The molecules on
the ends of equilibrium arrows are distinct molecules that can undergo a
chemical transformation to become the other. Resonance is not an
equilibrium in that the resonance structures don't actually exist on their own.
A resonance hybrid is not the average of resonance contributors that
fluctuate from one to the other.
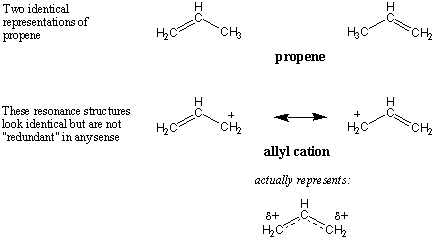
Resonance vs. Isomerism
In the next chapter we will discuss
isomers,
molecules that have
the same molecular formula but are different in some way. It is important
to note that resonance structures are not isomers of one another, simply
because they don't actually exist. Another common question is of the following
nature: The molecule propene can be written in two separate orientations, but
both represent the same molecule; in other words, the structures are identical.
Now consider the resonance structures of the allyl cation. Aren't these
identical too? And if so how can we consider them distinct resonance
structures?
Once again, the answer is that the resonance forms are only representations of
the true allyl cation; the resonance forms themselves don't exist. The
positioning of electrons on resonance structures is all-important. Seemingly
similar resonance forms are often necessary in order convey the true nature of
some molecule.
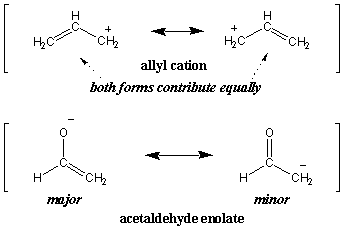
Major and Minor Resonance Contributors
In our earlier definition of resonance we said that the resonance hybrid is
a weighted average of its resonance forms. In fact, not all resonance
structures contribute equally. As an extension of the color analogy, imagine
that we try to describe a very dark shade of gray as a hybrid of white and
black. Clearly, dark gray resembles black much more than it resembles white.
Such dominant resonance forms are said to be major resonance structures,
whereas the less dominant forms are minor resonance structures. For instance,
both resonance forms of the allyl cation contribute equally (hence both are
major). However, the acetaldehyde enolate is dominated by the major form on the
left:
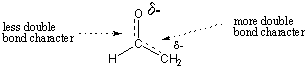
The ability to roughly assess the stability of organic molecules is an important
skill, and one that you will acquire as you increase your intuition of organic
chemistry. For the enolate example, we can use a simple electronegativity
argument to justify why the left form is more stable. Because oxygen is more
electronegative than carbon, the negative charge is stabilized more by residing
on
oxygen. Thus, in the resonance hybrid, we would expect oxygen to bear a larger
partial negative charge than carbon. We would also expect the C-C bond to have
greater double-bond character than the C-O bond.
Resonance Stabilization
We have seen that resonance structures can be used to describe molecules whose electrons are not fixed on specific atoms or bonds but are spread out over several atoms or bonds. This phenomenon is called electron delocalization. Delocalization is an energetically favorable process: by distributing charge over a greater volume, the net energy of the molecule is lowered. The result is that a resonance hybrid is more stable than any of its contributing resonance structures because of delocalization in the resonance hybrid. Hence, resonance hybrids are said to be resonance stabilized. An important guideline in organic chemistry is that a molecule becomes more stable whenever more resonance structures can be drawn for it. Additional resonance structures, even those relatively high in energy, always stabilize; they never destabilize. Of course, the more stable the additional resonance form is, the more stabilization the resonance hybrid receives.


 payment page
payment page



