Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews April 2, 2025 March 26, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Resonance
When drawing Lewis structures, sometimes you will find that there are many ways to place double bonds and lone pairs about a given framework of atoms. How does we decide whether one or another placement is correct? The answer, as it turns out, is neither and both. The actual arrangement of electrons in a given molecule is a weighted average of all the valid Lewis structures that can be drawn for that given atomic connectivity. The "real" molecule, the one that actually exists in the world, is said to be a resonance hybrid of all its contributing Lewis structures. Each Lewis structure that contributes to the resonance hybrid is a resonance structure.
The classical example of resonance is benzene, C6H6. Two good Lewis structures for benzene exist that differ only in their placement of double bonds. If either structure were correct, benzene would consist of alternating long single bonds and short double bonds. However, it has been determined experimentally that all six bonds on the ring are identical. The natural interpretation is that the three double bonds are distributed evenly around the ring, so that each bond has a bond order of one and a half.
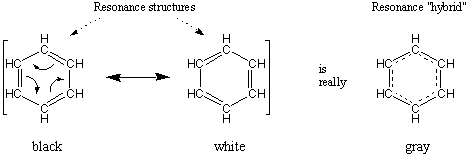
A double headed arrow is placed between resonance structures to denote them as such. In addition, sometimes we place all the resonance contributors within brackets for clarity.
It's important to remember that although the molecule described by resonance has characteristics of all its resonance contributors, it is fully neither one. For instance, the color gray might be described as being a resonance hybrid of white and black. And although gray takes on characteristics of both black and white, it would be incorrect to describe gray as being black or white.
Sometimes double-headed arrows are used to denote how one resonance structure
can be derived from another via the flow of electrons. This curved-arrow
formalism is a very useful bookkeeping tool that allows us to keep track of
the movement of pairs of electrons during reactions. The arrows are drawn from
the source of the electron motion, which can be a bonded electron pair or a lone
pair, to the destination of the electrons, typically an atom or a place between
two atoms. The figure below illustrates correct and incorrect usages of the
curved-arrow formalism. We will see that it is a very useful tool for
describing reaction mechanisms, the step-by-step processes by which
reactions occur. Such exercises are affectionately referred to as "arrow
pushing".

Resonance is such an important concept to master early on in your organic chemistry education that it's worthwhile to clear up two potential misunderstandings. Resonance and equilibrium, and resonance and isomerism are often confused.
Please wait while we process your payment

