Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews April 26, 2025 April 19, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Determining the Rate Law
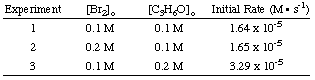
To calculate the order of the reaction for bromine, notice that experiments 1 and 2 hold the concentration of acetone constant while doubling the concentration of bromine. The initial rate of the reaction is unaffected by the increase in bromine concentration, so the reaction is zero order in bromine. We can prove this mathematically by taking the ratio of the rates from experiments 1 and two:
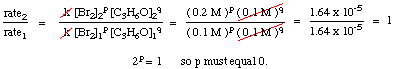
As you can see in the above equations, by holding the concentrations of all but one species constant between two experiments, you can calculate the order of the reaction in a single reactant at a time. By similar reasoning, we can conclude that because the rate of reaction doubled when the concentration of acetone was doubled (cf. experiments 1 and 3) the reaction must be first order in acetone. However, had the rate quadrupled or octupled with a doubling of the acetone concentration, the reaction would have been second or third order in acetone, respectively. In practice, you will likely never see a reaction with an order higher than 3. If you calculate an order higher than 3 for a reaction, double check your math because that is highly unusual. If you compute a fractional power for a reactant's order, do not be discouraged; they are quite common (especially half-order reactions).
To calculate the value of k, the rate constant, you simply plug into the rate law the values of the concentrations, the orders, and the rate of the reaction from any one of the three experiments. All three experiments should give a value of 1.64 x 10-4 s-1. You should prove this to yourself.
One major disadvantage to using the method of initial rates is the need to perform multiple experiments. Another disadvantage is that it works only for relatively slow reactions. If your reaction proceeds too fast, the rate that you measure will have a large range of uncertainty. To combat these problems, chemists have developed a method that uses data on concentration versus time during a reaction to infer the order of a reaction. The strategy works by comparing concentration versus time information to the mathematical predictions made by integrated rate laws. This is usually accomplished by assuming that a reaction has a certain order and making a plot out of the data that should be linear if the assumption about the rate law is correct. If we guess correctly, the graph is linear. If we are wrong, then the graph is curved and we need to choose another order against which to plot our data.
To practice this method, we will first need to know the forms of the integrated rate laws for some common reaction orders. Luckily, only orders zero through two are common so we need to only consider reactions with these three orders. Due to the mathematical complexities of the problem, we will only consider rate laws of the form rate = [A]n. However, with some experimental tricks I will explain below, that treatment does allow us to use the method of integrated rates for a reaction with any arbitrary rate law.
By integrating the differential rate law (the simple rate law from Heading ), we derive the integrated rate law for a reaction. Without proof, we present below the forms of the integrated rate laws for reaction orders zero through two:
Please wait while we process your payment

