Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment
Get instant, ad-free access to our grade-boosting study tools with a 7-day free trial!
Learn more



Create Account
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Log into your PLUS account
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
Select Your Plan
Monthly
$5.99
/month + tax
Annual
$29.99
/year + taxAnnual
2-49 accounts
$22.49/year + tax
50-99 accounts
$20.99/year + tax
Select Quantity
Price per seat
$29.99 $--.--
Subtotal
$-.--
Want 100 or more? Request a customized plan
Monthly
$5.99
/month + taxYou could save over 50%
by choosing an Annual Plan!

Annual
$29.99
/year + taxSAVE OVER 50%
compared to the monthly price!
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
Annual
$22.49/month + tax
Save 25%
on 2-49 accounts
Annual
$20.99/month + tax
Save 30%
on 50-99 accounts
| Focused-studying | ||
| PLUS Study Tools | ||
| AP® Test Prep PLUS | ||
| My PLUS Activity | ||
Testimonials from SparkNotes Customers
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Testimonials from SparkNotes Customers
No Fear provides access to Shakespeare for students who normally couldn’t (or wouldn’t) read his plays. It’s also a very useful tool when trying to explain Shakespeare’s wordplay!
Erika M.
I tutor high school students in a variety of subjects. Having access to the literature translations helps me to stay informed about the various assignments. Your summaries and translations are invaluable.
Kathy B.
Teaching Shakespeare to today's generation can be challenging. No Fear helps a ton with understanding the crux of the text.
Kay H.
Create Account
Select Plan
Payment Info
Start 7-Day Free Trial!
Payment Information
You will only be charged after the completion of the 7-day free trial.
If you cancel your account before the free trial is over, you will not be charged.
You will only be charged after the completion of the 7-day free trial. If you cancel your account before the free trial is over, you will not be charged.
Order Summary
Annual
7-day Free Trial
SparkNotes PLUS
$29.99 / year
Annual
Quantity
51
PLUS Group Discount
$29.99 $29.99 / seat
Tax
$0.00
SPARK25
-$1.25
25% Off
Total billed on Nov 7, 2024 after 7-day free trail
$29.99
Total billed
$0.00
Due Today
$0.00
Promo code
This is not a valid promo code
Card Details
By placing your order you agree to our terms of service and privacy policy.
By saving your payment information you allow SparkNotes to charge you for future payments in accordance with their terms.
Powered by stripe
Legal
Google pay.......



Thank You!
Your group members can use the joining link below to redeem their membership. They will be prompted to log into an existing account or to create a new account. All members under 16 will be required to obtain a parent's consent sent via link in an email.Your Child’s Free Trial Starts Now!
Thank you for completing the sign-up process. Your child’s SparkNotes PLUS login credentials are [email] and the associated password. If you have any questions, please visit our help center.Your Free Trial Starts Now!
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Sorry, you must enter a valid email address
By entering an email, you agree to our privacy policy.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment
Month
Day
Year
Please read our terms and privacy policy
Please wait while we process your payment

Galvanic Cells
Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. The key to gathering the electron flow is to separate the oxidation and reduction half-reactions, connecting them by a wire, so that the electrons must flow through that wire. That electron flow, called a current, can be sent through a circuit which could be part of any number of electrical devices such as radios, televisions, watches, etc.
The figure below shows two typical setups for galvanic cells. The left hand cell diagram shows and oxidation and a reduction half-reaction joined by both a wire and a porous disk, while the right hand cell diagram shows the same cell substituting a salt bridge for the porous disk.
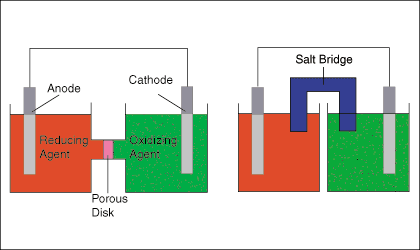
The salt bridge or porous disk is necessary to maintain the charge neutrality of each half-cell by allowing the flow of ions with minimal mixing of the half-cell solutions. As electrons are transferred from the oxidation half-cell to the reduction half-cell, a negative charge builds in the reduction half-cell and a positive charge in the oxidation half-cell. That charge buildup would serve to oppose the current from anode to cathode-- effectively stopping the electron flow--if the cell lacked a path for ions to flow between the two solutions.
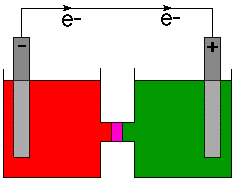
The above figure points out that the electrode in the oxidation half-cell is called the anode and the electrode in the reduction half-cell is called the cathode. A good mnemonic to help remember that is "The Red Cat ate An Ox" meaning reduction takes place at the cathode and oxidation takes place at the anode.
The anode, as the source of the negatively charged electrons is usually marked with a minus sign (-) and the cathode is marked with a plus sign (+). Physicists define the direction of current flow as the flow of positive charge based on an 18th century understanding of electricity. As we now know, negatively charged electrons flow in a wire. Therefore, chemists indicate the direction of electron flow on cell diagrams and not the direction of current. To make that point clear, the direction of electron flow is indicated on with a arrow and the symbol for an electron, e- .
Instead of drawing a cell diagram such as or chemists have devised a shorthand way of completely describing a cell called line notation. This notation scheme places the constituents of the cathode on the right and the anode components on the left. The phases of all reactive species are listed and their concentrations or pressures are given if those species are not in their standard states (i.e. 1 atm. for gasses and 1M for solutions). All phase interfaces are noted with a single line ( | ) and multiple species in a single phase are separated by commas. For example, a half-cell containing 1M solutions of CuO and HCl and a Pt electrode for the reduction of Cu2+ would be written as:
Please wait while we process your payment





