Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews February 6, 2025 January 30, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Electrolysis
Electroplating allows the production of metal coatings of such desirable commodities as silver and gold. People make fortunes gold or silver plating junk metal (usually aluminum) because they can sell gold plated necklaces for a comparable price to the real thing (or even pass them off as being solid gold). That's how electrochemistry can be used to rip you off! In our discussion of electroplating, we will discuss how you can set up a cell for electroplating, how you can calculate the amount of precious material consumed, and various other calculations you can perform with electroplating. In terms of the variety of electrochemistry problems possible to ask, this section, perhaps rivaled by Thermodynamics, is the richest.
The setup for electroplating is quite simple and the entire cell is usually conducted in a single solution as shown in .
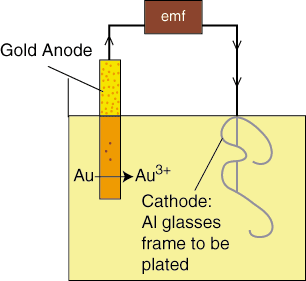
The gold from the anode is oxidized and dissolves in solution as Au3+. The electrons arriving at the aluminum glasses frame cathode reduce the Au3+ in solution to Au (s) on the surface of the frame cathode. We can calculate how long we should have our glasses frame in solution if we want a certain amount of gold to be plated.
Let's assume it takes 1.0 g of gold to provide an adequate coating for our glasses and also assume that we are using an emf sufficient to produce 10 amperes (A) of current (1 A = 1 coulomb per second). how long it will take to plate that 1.0 g of gold.
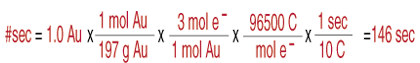
As you can see from the , such a problem only involves the use of unit cancellation. To calculate the time needed to deposit a certain amount of material, you need to start with the amount, converted to moles. Then, multiply by the number of electrons consumed in the reduction (in this case 3). Using the definition of a faraday, 96500 C per mole of electrons, you can convert between moles and charge. Finally, by using the definition of an ampere, 1 C per second, you can convert the amount of charge required to deposit the material into a time in seconds. There are various ways of phrasing this same problem such as "how much gold is deposited in 146 seconds at 10 A" or "what current is required to deposit 1.0 g of gold in 146 seconds." Don't be fooled by those permutations of the same problem, they all boil down to simple unit cancellation which you have been doing since you learned how to do stoichiometry. Also note that in these problems, you do not need to know the cell potential. Students often try, incorrectly, to use the cell potential somewhere in that calculation. Furthermore, you need only know the number of electrons transferred--you could solve the same problem without even knowing what material was being plated (as long as you know its molar mass).
Please wait while we process your payment

