Please wait while we process your payment
If you don't see it, please check your spam folder. Sometimes it can end up there.
If you don't see it, please check your spam folder. Sometimes it can end up there.
Please wait while we process your payment

By signing up you agree to our terms and privacy policy.
Don’t have an account? Subscribe now
Create Your Account
Sign up for your FREE 7-day trial
By signing up you agree to our terms and privacy policy.
Already have an account? Log in
Your Email
Choose Your Plan
Individual
Group Discount
Save over 50% with a SparkNotes PLUS Annual Plan!
 payment page
payment page
Purchasing SparkNotes PLUS for a group?
Get Annual Plans at a discount when you buy 2 or more!
Price
$24.99 $18.74 /subscription + tax
Subtotal $37.48 + tax
Save 25% on 2-49 accounts
Save 30% on 50-99 accounts
Want 100 or more? Contact us for a customized plan.
 payment page
payment page
Your Plan
Payment Details
Payment Summary
SparkNotes Plus
You'll be billed after your free trial ends.
7-Day Free Trial
Not Applicable
Renews January 28, 2025 January 21, 2025
Discounts (applied to next billing)
DUE NOW
US $0.00
SNPLUSROCKS20 | 20% Discount
This is not a valid promo code.
Discount Code (one code per order)
SparkNotes PLUS Annual Plan - Group Discount
Qty: 00
SparkNotes Plus subscription is $4.99/month or $24.99/year as selected above. The free trial period is the first 7 days of your subscription. TO CANCEL YOUR SUBSCRIPTION AND AVOID BEING CHARGED, YOU MUST CANCEL BEFORE THE END OF THE FREE TRIAL PERIOD. You may cancel your subscription on your Subscription and Billing page or contact Customer Support at custserv@bn.com. Your subscription will continue automatically once the free trial period is over. Free trial is available to new customers only.
Choose Your Plan
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
For the next 7 days, you'll have access to awesome PLUS stuff like AP English test prep, No Fear Shakespeare translations and audio, a note-taking tool, personalized dashboard, & much more!
You’ve successfully purchased a group discount. Your group members can use the joining link below to redeem their group membership. You'll also receive an email with the link.
Members will be prompted to log in or create an account to redeem their group membership.
Thanks for creating a SparkNotes account! Continue to start your free trial.
We're sorry, we could not create your account. SparkNotes PLUS is not available in your country. See what countries we’re in.
There was an error creating your account. Please check your payment details and try again.
Please wait while we process your payment

Your PLUS subscription has expired
Please wait while we process your payment
Please wait while we process your payment

Problems and Solutions
Problem :
What is the pH of a solution of 0.36 M HCl, 0.62 M NaOH, and 0.15 M HNO3?
Hydrochloric acid and nitric acid are strong acids, and sodium hydroxide is a strong base; these all dissociate completely. The total [H+] from the two acids is 0.51 M and [OH-] from NaOH is 0.62 M. Therefore, 0.51 moles per liter of H+ will react with 0.51 moles per liter of OH- to form water. That leaves a 0.11 M NaOH solution. The pOH of a 0.11 M NaOH solution is 0.96 pOH units, and the pH is 13.04 pH units.
Problem :
What percent of formic acid (HCOOH) is dissociated in a 0.1 M solution of formic acid? The Ka of formic acid is 1.77 x 10-4.
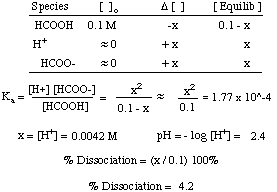
Problem :
What happens to the pH of a 0.1 M solution of formic acid when enough HCl (g) is added to make the solution 0.01 M in HCl?
Note that in this problem the volume of the solution does not change so you do not have to recalculate the concentration of formic acid. The only change to the problem is that there is now an initial concentration of H+ in solution:
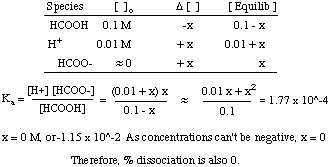
As you can see, the addition of a strong acid can, by Le Chatlier's principle, cause a weak acid not to dissociate. An exact solution for this problem does show a small dissociation of formic acid that is insignificant.
Problem :
What is the pH of a 0.001 M solution of H2SO4? HSO4- has a pKa of 1.2 x 10- 2.
To solve this problem, you must first note that sulfuric acid's first deprotonation is as a strong acid, so we have a concentration of 0.001 M H+ to start and 0.001 M hydrogen sulfate. Because hydrogen sulfate is a weak acid, this problem becomes very similar to the last one (see ). The calculated value for pH is 2.73 pH units (note that it is lower than 3 due to the dissociation of hydrogen sulfate).
Please wait while we process your payment

